Anvisa makes recommendations for using Pfizer's vaccine in children

After authorizing the use of the Pfizer vaccine in children aged between 5 and 11 years , the National Health Surveillance Agency (Anvisa) presented today (16) some recommendations and conditions that must be observed by health authorities for the immunization of this public. According to the agency, attention must be redoubled since both the dose and the formulation of the vaccine to be applied will be different from those applied to young people and adults.

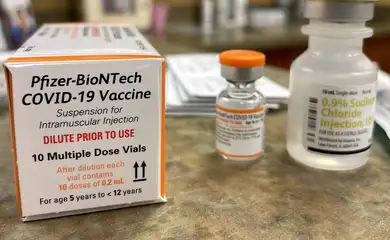
As in the other groups, vaccination of children should prioritize groups considered to be at risk. A very important point, highlighted by the director of Anvisa and rapporteur of the drug release process, Meiruze Sousa Freitas, is that parents or guardians be aware of the vaccine bottle, which will be orange. For adults, the bottle is purple.
Administration of the vaccine in children will be two doses of 10 micrograms three weeks apart. “The volume to be applied is 0.2 ml in a 1 ml syringe”, explained the director.
Recommendations
Meiruze recalled that it will be up to the Ministry of Health to decide on "convenience and opportunity" to include the vaccine in the National Immunization Program, but it is up to Anvisa to present the recommendations and conditions that must be followed for the vaccination of children in this age group.
"The vaccination of children in this age group should start after complete training of the health teams that will apply it, since the vast majority of adverse events after vaccination are due to the administration of the wrong product at the wrong age groups, of doses inadequate and erroneous preparation of the product”, said the director.
Another recommendation from Anvisa is that the vaccination of children be carried out in a “specific and segregated environment from the vaccination of adults”. The environment must be “welcoming and safe for the pediatric population”. It is also recommended that children remain in the place where the vaccination takes place for at least 20 minutes after application, in order to be observed during this period.
The room where the vaccine will be administered must be exclusive for the application of that vaccine. And it should not be used for the application of other vaccines, even pediatric ones. If there is no such possibility in the infrastructure, for this application, all care should be taken with a view to safe administration.
In the case of isolated communities, such as indigenous villages, Anvisa recommends that, whenever possible, the vaccine be made on separate days, not coinciding with the days of application in adults.
15 day interval
According to the director of Anvisa, the vaccine should not be administered concomitantly with other vaccines in the children's calendar. “As a precaution, a 15-day break is recommended,” said the director.
The drive thr u vaccination modality should also be avoided.
Another recommendation is that health workers should inform parents or guardians who accompany children and adolescents about symptoms and expected reactions after vaccination, such as pain, swelling or local redness, fever, fatigue, headache or lymphadenopathy (ganglia) in the armpit the arm that received the vaccine.
"Parents or guardians should seek a doctor if the child presents sudden chest pain, shortness of breath or palpitations after applying the vaccine," said the director. Children who turn 12 in the interval between the first and second dose should maintain the pediatric dose.
Health Secretaries
The National Council of Health Secretaries (Conass) released a note in which it expressed support for the approval of the immunizing agent for this audience. In it, the organization's president, Carlos Lula, highlights that the immunizing agent has already been approved for the age group by the European Medicines Agency (EMA), the American Food and Drug Administration (FDA) and the Israeli government.
"Given that to start vaccination in this age group it will be necessary to specifically formulate this vaccine with one third of the standard formula [10 micrograms per dose], Conass is awaiting the Ministry of Health's position regarding its acquisition, which is its competence. We also look forward to the evaluation process of the CoronaVac vaccine, by the Butantan Institute, for the vaccination of children and adolescents from 5 to 17 years old, already widely used in other countries, with immediate availability in Brazil”, said Carlos Lula.
Contacted by Agência Brasil , the Ministry of Health informed that there is still no forecast on when it will start to apply the Pfizer vaccine in children aged between 5 and 11 years.
threats
The CEO of Anvisa, Antônio Barra Torres, took advantage of the announcement to denounce that all directors of Anvisa received threats - some of death - from people against vaccination in children.
According to Torres, "the intensification of anti-vaccine violence is on the rise", but the work being developed by the agency will not be harmed.
He informed that "it is not up to Anvisa, but to the health authorities, the application of the immunizing agent".
Text translated using artificial intelligence.