COVID-19: Brazil steps up vaccination, monitors new variants

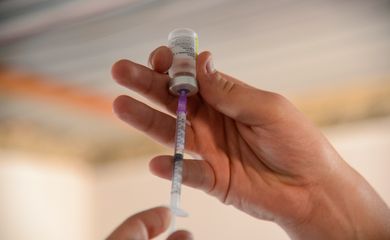
Following the identification of two subvariants of COVID-19 in Brazil (JN.1 and JG.3), the Ministry of Health announced it is monitoring the situation and has reinforced vaccination as the main means of protection against the disease.

“At this time, it is important that all Brazilians keep up to date with the vaccination scheme with all the doses recommended for each age group, including the bivalent booster,” the ministry stated. “All the vaccines currently available under SUS [Brazil’s national network of public health care services] are effective against the variants circulating in the country, preventing serious symptoms and deaths.”
Antiviral
In a statement, the ministry underscored that the antiviral nirmatrelvir/ritonavir is available under SUS for the treatment of COVID-19 infection in elderly people aged 65 and over and immunosuppressed people aged 18 and over, as soon as symptoms appear and there is confirmation of a positive test.
Subvariants
The JN.1 subvariant, initially detected in Ceará, has been gaining global proportions and now accounts for 3.2 percent of cases registered worldwide, the ministry reported. The JG.3 subvariant, also identified in Ceará, is being monitored in the states of São Paulo, Rio de Janeiro, and Goiás.
Both subvariants have been found in 47 countries, as per a report by the World Health Organization.
“The Ministry of Health remains aligned with all the scientific evidence, with the most up-to-date recommendations of the World Health Organization for tackling COVID-19, including planning for vaccination in 2024, which is underway.”
Planning for 2024
In October, the ministry announced the inclusion of the paediatric COVID-19 vaccine in the national vaccination program as well as the immunization of the population at high risk of worsening the disease, starting in 2024. The ministry guarantees it will have enough stock for both groups next year.
“The acquisition of vaccines for next year’s calendar is underway. The new contract stipulates the supply of the most up-to-date versions of the vaccines, provided they have been approved by the National Health Surveillance Agency.”